Features at-a-glance
Built-in timer - Display of time remaining before a measurement is taken. Ensures that all readings are taken at the appropriate reaction intervals for the test being performed.
Zero key - A simple press of the zero key on the face of the meter will account for the color and imperfections in the wine sample before reagent addition.
Auto shut-off - Automatic shut-off after 15 minutes of non-use when the meter is in measurement mode. Prevents wastage of batteries in the event the meter is accidentally left on.
Battery status indicator - Indicates the amount of battery life left.
Error messages - Messages on display alerting to problems including no light, inverted sample, and out of range.
Units of measure - Appropriate unit of measure is displayed along with reading.
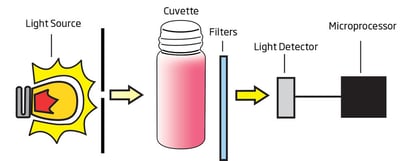
Tartaric acid concentrations in wine range normally from 1.5 to 4.0 g/L. This acid concentration should not be confused with total or titratable acidity of wines, which are often expressed as tartaric acid content as well. Although it is the tartaric acid that is the predominant acid present (up to 60% of the total acidity), others like malic, citric, and several volatile acids contribute significantly to total acidity. The HI83748 uses a method with two reagents to determine the concentration of tartaric acid less than 5.0 g/L (ppt). When both reagents are added to a sample containing tartaric acid, the sample will turn an orange-red hue; the greater the concentration, the deeper the color. The associated color change is then colorimetrically analyzed according to the Beer-Lambert Law. This principle states that light is absorbed by a complementary color, and the emitted radiation is dependent upon concentration. For the determination of reducing sugars, a narrow band interference filter at 525 nm (green) allows only green light to be detected by the silicon photodetector and omits all other visible light emitted from the tungsten lamp. As the change in color of the reacted sample increases, the absorbance of the specific wavelength of light also increases, while transmittance decreases.
Tartaric acid and tartrate play an important role in the stability of wines. They can be present in wine and juice in various forms, like tartaric acid (H2T), potassium bitartrate (KHT) or calcium tartrate (CaT). The ratio of these depends mainly on the pH of the wine. The percentage of tartrate present as bitartrate (HT-) is maximum at pH 3.7.
The formation of crystalline deposits (tartrate casse) is a phenomenon of wine aging that does not meet customer acceptance. It is therefore important to test for and reduce the potential for bottle precipitation. For example, by adjusting the pH of the wine, winemakers can significantly influence the potential of casse formation.
Potassium concentrations in wine can range from 600 to 2500 ppm in certain red wines. Although the potassium bi-tartrate is soluble in water, alcohol and low temperatures decrease its solubility. Especially during the alcoholic fermentation potassium bi-tartrate becomes increasingly insoluble resulting in supersaturation and precipitation. The KHT stability can be restored by chilling (with or without seeding). Wines with initial pH values below 3.65 can show a reduction in pH during cold stabilization because of generation of one free proton for each KHT precipitated. The pH may drop as much as 0.2 pH units. For wines at higher pH than 3.7 the pH shifts to a higher pH.
Calcium concentrations can range from 6 to 165 ppm and may complex with tartrate or oxalate to form crystalline precipitates. Calcium tartrate instabilities occur normally from 4 to 7 months after fermentation and are temperature independent.
Sulphates, proteins, gum, and poly-phenols can form stable complexes with tartrate thus inhibiting case formation. The complexes are mainly between poly-phenols and tartaric acid in red, and proteins in white wine. This explains why, as pigment polymerization occurs, the holding capacity of tartaric acid diminishes, resulting in delayed casse. The sulfate instead does complex with potassium from 50% in white wines up to 100% in red ones.
Tartaric acid concentrations in wine range normally from 1.5 to 4.0 g/L. This acid concentration should not be confused with total or titratable acidity of wines, which are often expressed as tartaric acid content as well. Although it is the tartaric acid that is the predominant acid present (up to 60% of the total acidity), others like malic, citric, and several volatile acids contribute significantly to total acidity. The HI83748 uses a method with two reagents to determine the concentration of tartaric acid less than 5.0 g/L (ppt). When both reagents are added to a sample containing tartaric acid, the sample will turn an orange-red hue; the greater the concentration, the deeper the color. The associated color change is then colorimetrically analyzed according to the Beer-Lambert Law. This principle states that light is absorbed by a complementary color, and the emitted radiation is dependent upon concentration. For the determination of reducing sugars, a narrow band interference filter at 525 nm (green) allows only green light to be detected by the silicon photodetector and omits all other visible light emitted from the tungsten lamp. As the change in color of the reacted sample increases, the absorbance of the specific wavelength of light also increases, while transmittance decreases.


