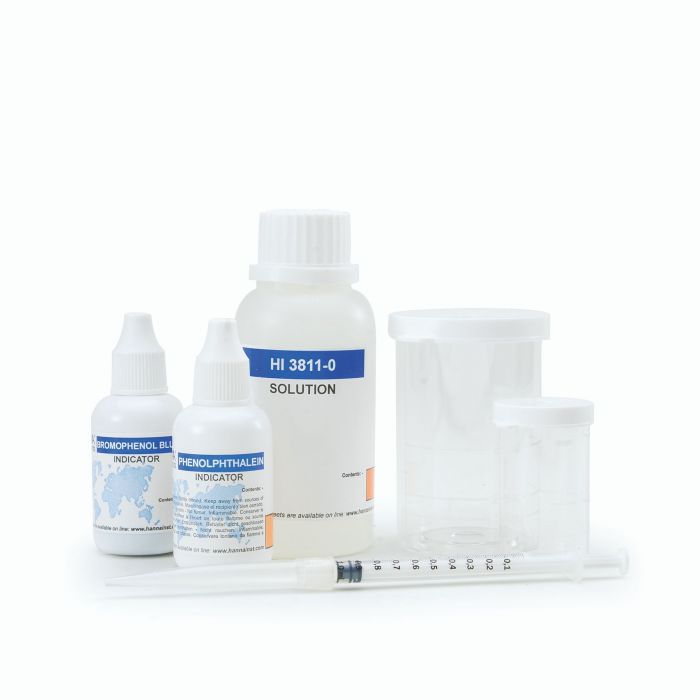
Alkalinity Chemical Test Kit - HI3811
- All reagents marked with expiration date and lot number for traceability
- Manual titration performed with color indicator
The HI3811 is a titration-based chemical test kit that determines the alkalinity concentration in samples within a 0 to 100 mg/L (ppm) CaCO3 or 0 to 300 mg/L CaCO3 range. The HI3811 is supplied with all of the necessary reagents and equipment to perform the analysis. The test kit contains enough reagents for perform approximately 110 tests.
Features at-a-glance
Complete setup
- All required materials are included with the test kit, such as the sample beakers, plastic syringe, phenolphthalein indicator, and bromophenol blue indicator.
High resolution
- Readings from 0 to 100 mg/L are determined to 1 mg/L resolution
- Readings from 0 to 300 mg/L are determined to 3 mg/L resolution
Replacement reagents available
- There is no need to buy a new kit when reagents are exhausted. The HI3811-100 reagent set can be ordered to replace the reagents supplied with the kit. This reagent set comes with 1 (10mL) dropper bottle of phenolphthalein indicator, 1 (10mL) dropper bottle of bromophenol blue indicator, and 1 (120mL) bottle of HI3811-0 reagent.
Significance of Use
Alkalinity is the quantitative capacity of a water sample to neutralize an acid to a set pH. This measurement is very important in determining the corrosive characteristics of water due primarily to hydroxide, carbonate, and bicarbonate ions. Other sources of alkalinity can be from anions that can be hydrolyzed such as phosphates, silicates, borates, fluoride, and salts of some organic acids. Alkalinity is critical in the treatments of drinking water, wastewater, boiler and cooling systems, and soils.
Alkalinity can be measured as Phenolphthalein Alkalinity and Total Alkalinity. The Phenolphthalein Alkalinity is determined by neutralizing the sample to a pH of 8.3 using a dilute hydrochloric acid solution and a phenolphthalein indicator. This process converts hydroxide ions to water, and carbonate ions to bicarbonate ions:
OH- + HCl → H2O + Cl-
CO32- + HCl → HCO3- + Cl-
Since bicarbonate ions can be converted to carbonic acid with additional hydrochloric acid, the Phenolphthalein Alkalinity measures total hydroxide ions, but only half of the bicarbonate contribution. To completely convert the carbonate ions, hydrochloric acid is added until the sample pH is 4.5, which is known as Total Alkalinity:
HCO3- + HCl → H2CO3 + Cl-
Specifications
|
Type |
titration |
|
Smallest increment |
1 mg/L (ppm) 3 mg/L (ppm) |
|
Range |
0-100 mg/L (ppm) 0-300 mg/L (ppm) |
|
Avg. number of tests |
110 |
|
Method |
phenolphthalein/bromphenol blue |
|
Ordering info |
HI3811 test kit comes with 10 mL phenolpthalein indicator, 10 mL bromophenol blue indicator, 120 mL alkalinity titrant, 10 mL calibrated vessel, 50 mL calibrated vessel, and calibrated syringe with tip. |